CAR T Cells: A Revolutionary Approach to Cancer Treatment
Just a decade ago, immunotherapy was considered a promising new avenue for treating specific types of advanced cancer. Today, it’s a cornerstone of cancer care. One particularly exciting form of immunotherapy is CAR T-cell therapy, which harnesses the body’s own immune system to fight cancer.
While not a cure-all, CAR T-cell therapies have shown remarkable success in some patients with advanced cancers, sometimes completely eradicating the disease for extended periods. Unlike other immunotherapies or cancer treatments, CAR T-cell therapies are personalized, utilizing a patient’s own T cells, the body’s natural defense against infected and diseased cells.
The Food and Drug Administration (FDA) approved the first CAR T-cell therapy in 2017 to treat children with acute lymphoblastic leukemia (ALL). Since then, several more have been approved for adults with blood cancers such as non-Hodgkin lymphoma and multiple myeloma. However, early on, some researchers questioned if CAR T-cell therapy would become more than a niche treatment for a select few, according to NCI’s Steven Rosenberg, M.D., Ph.D., a pioneer in immunotherapy and CAR T-cell therapy. Now, Dr. Rosenberg states that “[CAR T cells] have become a part of modern medicine.”
How CAR T-Cell Therapy Works: A “Living Drug”
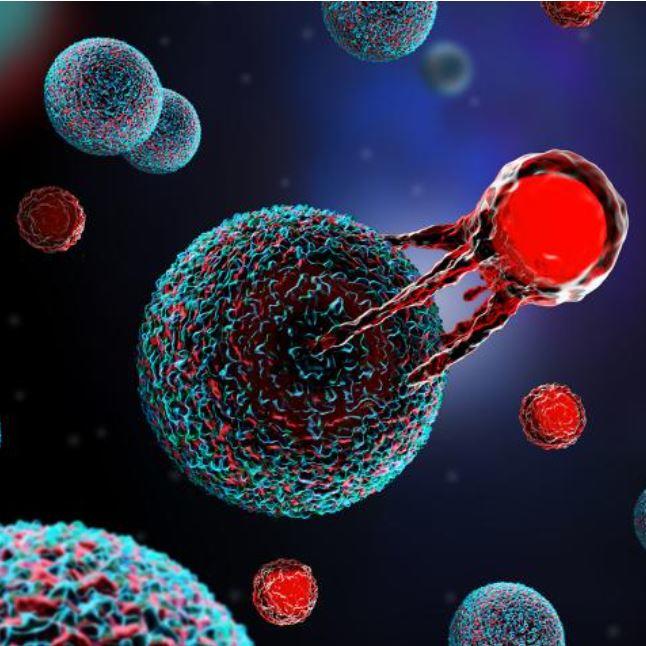
T cells are the foundation of CAR T-cell therapy. “We are giving patients a living drug,” explained Renier J. Brentjens, M.D., Ph.D., from Roswell Park Comprehensive Cancer Center in Buffalo, NY, and another early leader in the field. The process begins by collecting blood from the patient and isolating their T cells. These cells are then sent to a laboratory where they are genetically engineered to produce special proteins on their surface called chimeric antigen receptors, or CARs. These CARs enable the T cells to latch onto particular proteins, called antigens, found on cancer cells, and also boost the T cells’ ability to kill them.
Next, these modified T cells are grown, or “expanded,” into the hundreds of millions. This final CAR T-cell product is then sent back to the hospital to be infused back into the patient. The entire process, from blood collection to infusion, typically takes 3 to 5 weeks. Following the infusion, the T cells multiply within the patient’s body and, guided by their special receptors, target and destroy cancer cells displaying the specific antigen.
CAR T-Cell Therapies for Adults and Children
Prior to CAR T-cell therapy, standard chemotherapy cured more than 80% of children with B-cell ALL. However, there were few effective treatments for those whose cancers returned after chemotherapy or a stem cell transplant. The initial approval of tisagenlecleucel (Kymriah) was based on clinical trials demonstrating its ability to eliminate leukemia in most children with relapsed ALL. Long-term studies have shown that many of these children remain cancer-free for years, suggesting a potential cure. This treatment, also known as tisa-cel, is now a recommended standard for children with ALL that has relapsed after multiple other treatments.
Subsequent approvals extend to adults with blood cancers, including multiple myeloma and some forms of lymphoma. Again, these approvals came from large clinical trials that proved these treatments could eliminate advanced cancers in many patients, and even lead to apparent cures. These treatments are transformative for many adults, explains NCI’s James Kochenderfer, M.D., who led several CAR T-cell therapy trials. Patients with advanced lymphomas that aren’t controlled by other treatments have especially benefited from CAR T-cell therapies, according to Dr. Kochenderfer, who stated that before these therapies, a lot of these patients “were virtually untreatable.”
For example, in a clinical trial involving patients with advanced follicular lymphoma, the CAR T-cell therapy axi-cel (Yescarta) eliminated the cancer in almost 80% of participants. According to this trial, the disease hadn’t returned for many of these patients three years later. Further, in clinical trials focusing on people with large cell lymphoma, the most common form of the disease, over 30% of those treated were alive without any evidence of cancer five years after treatment.
FDA-Approved CAR T-cell Therapies
Here is a list of FDA-approved CAR T-Cell therapies, with their approved uses:
- Abecma (ide-cel): Multiple myeloma
- Aucatzyl (obe-cel): B-cell ALL (adult)
- Breyanzi (liso-cel): Follicular lymphoma, Large B-cell lymphoma, Mantle cell lymphoma, Chronic lymphocytic leukemia
- Carvykti (cilta-cel): Multiple myeloma
- Kymriah (tisa-cel): B-cell ALL (pediatric/young adult), Diffuse Large B-cell Lymphoma, Follicular lymphoma
- Tecartus (brexu-cel): B-cell ALL (adult), Mantle cell lymphoma
- Yescarta (axi-cel): Large B-cell lymphoma, Follicular lymphoma
Managing Side Effects of CAR T-Cell Therapies
Like all cancer treatments, CAR T-cell therapies can have severe side effects. Infections and a mass die-off of antibody-producing B cells are common. Two additional side effects of concern are cytokine release syndrome (CRS) and neurologic problems collectively called immune effector cell–associated neurotoxicity syndrome (ICANS), explained NCI’s Jennifer Brudno, M.D., who studies CAR T-cell therapies for blood cancers.
In CRS, infused T cells flood the bloodstream, releasing cytokines, which are chemical messengers that stimulate the immune response. The overabundance of cytokines in CRS can cause dangerously high fevers and sharp drops in blood pressure. In rare cases, severe CRS can be fatal. However, both children and adults may have CRS managed with tocilizumab (Actemra) and, if needed, steroids, Dr. Brudno said. Tocilizumab, originally used to treat inflammatory conditions like juvenile arthritis, blocks the activity of IL-6, which is a cytokine often secreted in large amounts by immune cells.
ICANS can manifest as confusion, excessive sleepiness, and speech impairments. Steroids are often used to treat ICANS, and when steroids aren’t completely effective, anakinra (Kineret), an antibody drug for rheumatoid arthritis, has been shown to be an effective option. Other studies suggest that administering anakinra soon after CAR T-cell therapy may prevent or reduce the severity of ICANS.
Progress and Challenges for Solid Tumors
In contrast to the advances made in CAR T-cell therapies for blood cancers, progress for those targeting solid tumors has lagged. Researchers are still working to identify antigens that are present on solid tumor cancer cells but not healthy cells. Another significant hurdle is the immunosuppressive environment within and around tumors. For example, tumor cells and other parts of the immune system produce molecules that can make CAR T cells malfunction or restrict their access to the tumor.
However, a major obstacle is tumor heterogeneity, according to Crystal Mackall, M.D., director of the Parker Institute for Cancer Immunotherapy at Stanford University. Solid tumors of the same cancer type can vary greatly at the molecular level from patient to patient, and even within the same patient. For example, some tumor cells may not have targetable antigens, or not enough for CAR T cells to fully function.
Despite the challenges, there is hope for the future of treating solid tumors. Dr. Mackall’s team at Stanford reported promising results from a small clinical trial of a CAR T-cell therapy in select children and young adults with diffuse midline glioma, a deadly brain cancer. Additionally, another team has reported encouraging findings utilizing a different CAR T-cell therapy in these children. Successful outcomes have also resulted from small clinical trials testing CAR T-cell therapies in patients with other solid cancers, including ovarian and colorectal cancers.
“I think all of us in this field know that we’re just scratching the tip of the iceberg about what we can do with regard to engineering these CAR T cells,” stated Dr. Mackall. “There are many, many next-generation approaches to the problems that are limiting [their effectiveness] in solid tumors.”
The Future of CAR T-Cell Therapy
Research into CAR T-cell therapy is accelerating rapidly. Researchers have developed CAR T-cell therapies designed to have fewer side effects and that could be used for any type of blood cancer. Some of these newer forms of CAR T-cell therapies are already being studied in small clinical trials. Furthermore, large clinical testing is taking place where CAR T-cell therapies are using T cells collected from healthy donors, rather than individual patients. By using donated, or allogeneic, T cells, these CAR T-cell therapies can be made in advance, like traditional cancer drugs. This means they can be immediately available as “off-the-shelf” treatments, as opposed to the current manufacturing process, which can take weeks. However, because these therapies use donated T cells, additional genetic engineering is needed to stop the patient’s immune system from perceiving and attacking the T cells.
Researchers are now investigating the implementation of CAR T-cell therapy earlier in the treatment process, rather than reserving it until multiple other treatments have failed. The approved uses of two CAR T-cell therapies have already been expanded to treat adults with some blood cancers after the initial treatment has stopped working, which is known as second-line treatment. This strategy is also being tested in children. For example, one clinical trial is testing a CAR T-cell therapy as a second-line treatment for children with “high-risk” B-cell ALL for whom the standard initial chemotherapy hasn’t fully eliminated their cancer after 6 months.
Terry Fry, M.D., who has led CAR T-cell therapy trials at Children’s Hospital Colorado, says, “Patients who respond well ‘could be spared 2 more years of chemotherapy.’ That’s amazing to think about.”