Enhancing CAR-T Cell Therapy’s Effectiveness
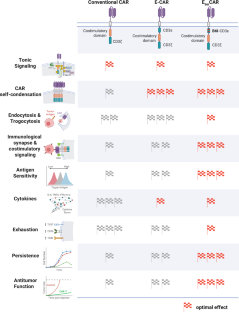
Chimeric antigen receptor (CAR)-T cell therapy has emerged as a revolutionary method in cancer treatment. However, the long-term efficacy and widespread application of this therapy are limited by factors like antigen insensitivity, poor persistence, and T-cell exhaustion. Researchers, including Xu et al., recently unveiled a novel CAR engineering strategy to overcome these challenges. This strategy involves incorporating a modified CD3ε intracellular domain, known as EB6I, into the conventional CAR structure [1].
This novel modification enhances antigen sensitivity and boosts the cytotoxic activity of CAR-T cells. The results of preclinical trials demonstrate promising efficacy in the treatment of various tumor models, including both solid tumors and hematological malignancies, thus underscoring the potential of this approach. The strategy can be applied to different CAR types, such as 28Z and BBZ CARs, suggesting its transformative potential in overcoming the limitations of current CAR-T-cell immunotherapy options.
CAR-T Cells: How They Work
CARs are synthetic receptors engineered to integrate the extracellular antigen-binding domain of antibodies with intracellular signaling domains derived from the T-cell receptor (TCR) and costimulatory receptors. By genetically engineering T cells to express these CARs, scientists create CAR-T cells capable of targeting and eliminating tumor cells expressing specific tumor-associated antigens, regardless of MHC presentation [2]. This modular design effectively reprograms T-cell specificity and function, thus forming the molecular basis for CAR-T-cell immunotherapy.
CAR-T cell therapy has shown significant success in treating relapsed or refractory B-cell malignancies, including acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL), and multiple myeloma. For these hematologic malignancies, CD19-targeted CAR-T cell therapies, such as the FDA-approved Kymriah (tisagenlecleucel) and Yescarta (axicabtagene ciloleucel), are common in clinical settings [3]. Recent studies have also demonstrated the potential of CAR-T cell therapy for treating B-cell- and autoantibody-mediated autoimmune diseases, including lupus, necrotizing myopathy, and diffuse cutaneous systemic sclerosis [4, 5].
Challenges in Treating Solid Tumors
Despite its successes, CAR-T cell therapy faces significant obstacles when treating solid tumors. The primary challenges are tumor antigen heterogeneity and the immunosuppressive tumor microenvironment (TME). Unlike native TCRs, conventional CARs often exhibit poor antigen sensitivity and suboptimal coordination of intracellular signaling molecules. This limitation stems largely from their inability to form mature immune synapses (ISs) that incorporate multiple signaling pathways. As a result, CAR-T cells frequently fail to target tumor cells with low antigen expression and are susceptible to functional exhaustion, thereby compromising their long-term effectiveness [6]. Consequently, optimizing the signal transduction capabilities of CARs is critical to overcoming the current limitations of CAR-T cell therapy [7].
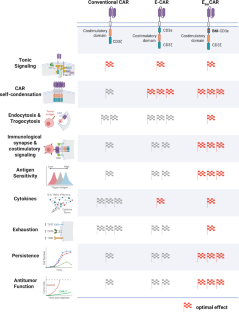
Figure 1. (The original figure was not described, so this is used as a placeholder. Insert the actual graph here)
Future Directions
The study’s findings offer an innovative approach to improving CAR-T cell therapy, addressing the limitations of conventional methods. Incorporating the EB6I domain into CAR structures enhances antigen sensitivity and sustains cytotoxic activity. Further research could explore this strategy in other contexts, focusing on optimizing the signaling dynamics of CARs to improve therapeutic outcomes [7]. Continued advancements are essential to developing CAR-T cell immunotherapy with enhanced efficacy and broader applicability.
References
- Xu X, Chen H, Ren Z, Xu X, Wu W, Yang H, et al. Phase separation of chimeric antigen receptor promotes immunological synapse maturation and persistent cytotoxicity. Immunity. 2024;57:2755–71.e8.
- Wu L, Wei Q, Brzostek J, Gascoigne N. Signaling from T-cell receptors (TCRs) and chimeric antigen receptors (CARs) on T cells. Cell Mol Immunol. 2020;17:600–12.
- Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378:439–48.
- Jin X, Xu Q, Pu C, Zhu K, Lu C, Jiang Y, et al. Therapeutic efficacy of anti-CD19 CAR-T cells in a mouse model of systemic lupus erythematosus. Cell Mol Immunol. 2021;18:1896–903.
- Wang X, Wu X, Tan B, Zhu L, Zhang Y, Lin L, et al. Allogeneic CD19-targeted CAR-T therapy in patients with severe myositis and systemic sclerosis. Cell. 2024;187:4890–904.e9.
- Sterner RC, Sterner RM. CAR-T-cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69.
- Duan Y, Chen J, Meng X, Liu L, Shang K, Wu X, et al. Balancing activation and costimulation of CAR tunes signaling dynamics and enhances therapeutic potency. Mol Ther. 2023;31:35–47.